
This is a cooperative effort of our period 3 class to document what occurs in class on a daily basis. This is "our book", written by us, for us (and for whomever else stops by). Each day, one student is the "scribe". Before the next class, that student "adds a post" in which he/she explains what happened in class. Concepts must be explained and documented. Examples, diagrams, graphs, scanned worksheets, links, photos or videos (taken with a camera or cell phone) can be included.
Thursday, September 30, 2010
Thursday, September 30

Wednesday, September 29, 2010
Wednesday, September 29
He then told us to get our lab notebooks out. We were to do Mendeleev for a Day Lab (AMI4) and the Oleic Acid Lab (AMI3). He summarized what we were supposed to do while we wrote down the purposes of the labs.
He told us to find a partner, or if we preferred, work alone to do this lab. Mendeleev for a Day is a lab that required the sheet that was given to us yesterday. It had 20 squares and we were supposed to cut them out for class. Each square had the Atomic Mass, the melting point (degrees C), the boiling point (degrees C), the number of O in oxide, and the number of Cl in chloride. Those were two chemical properties of the element and also two physical properties. We were to start putting the squares in order from least to greatest according to the atomic mass in a row. From there, you were to look for common points, and to move them into columns. More specifically, the number of O in oxide and the number of Cl in chloride. These columns are called families. Mr. H gave an example of his own family. Mr. H's father had a specific way of walking. If you look at him and then Mr. H, you wouldn't see a difference. He also gave us an example of the males in his family get gray h
 airs around their 30's. He told us this because it relates to the lab. Families have similarities and in the periodic table, the columns have similar properties, putting them in the same "family." Anyways, after we put them in columns, we taped them into the Data section in our lab notebooks. Remember to put the squares on the paper landscaped. This provides more room for the squares. Or, you could put it across two pages in your lab notebook like this (left):
airs around their 30's. He told us this because it relates to the lab. Families have similarities and in the periodic table, the columns have similar properties, putting them in the same "family." Anyways, after we put them in columns, we taped them into the Data section in our lab notebooks. Remember to put the squares on the paper landscaped. This provides more room for the squares. Or, you could put it across two pages in your lab notebook like this (left):
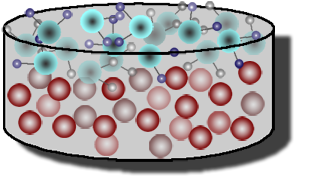
The Oleic Acid Lab is much different. We were working with lycopodium powder. Lycopodium powder is made out of a type of moss, and can trigger some allergic reactions, if you are allergic. Oleic acid does not mix with water, but dissolves the lycopodium powder. The first thing we did was put our safety goggles on. At our lab stations, there was already a yellow tray with water. We were to gently put the lycopodium powder on the surface of the water until it looked a little filmy. Mr. H did a little demonstration on how to do it:
This is how it looked like (bottom):
Then, when we
 were ready, Mr. H came around each lab group to put a drop of oleic acid into the tray. What we observed was that the oleic acid dissolved in the lycopodium powder, but in the process, it spread out on top of the water. It had a large diameter. This is what the oleic
were ready, Mr. H came around each lab group to put a drop of oleic acid into the tray. What we observed was that the oleic acid dissolved in the lycopodium powder, but in the process, it spread out on top of the water. It had a large diameter. This is what the oleicacid looked like after Mr. H put it in the tray. As you can see from the picture, it dissolved the lycopodium powder and made the water easier to see through. There is no more of that filmy look.
Mr. H gave us a sheet of paper to record our data in, and the number of drops/mL of .500% oleic acid solution is 32. We decided that the average diameter of the drop was 14.8 cm. You are to find the surface area, height, length, width, volume, and mass. For homework, we are to finish the data sheet and the conclusion/discussion for both labs. See you tomorrow!
Tuesday, September 28, 2010
Tuesday September 28, 2010.
 Today Mr. Henderson started out the class by reviewing last nights scribe's blog. He went over all the plus points of it and went into further detail on how the blog reviewed what we did in class the previous day. He also talked about the 5 postulates. He then told us a little about what we were going to do today in class. Today in class our main goal was to learn about the atomic structure, atomic number, and the mass number.He then started to review page one in the packet. We went over most of it yesterday but we finished up today. We talked about how the Dalton theory of an atom was just a circle with the element name inside. There was no proposed structure to it. The JJ Thomson theory of an atom was that it looked like plum pudding. The Rutherford theory was that positive charges were concentrated in the nucleus which was in the center of the atom and then the electrons surrounded the empty space outside the nucleus. Bohr's theory of the atomic structure was that electrons are in orbit around the nucleus. Bohr's structure also only worked for hydrogen.The picture on the left shows these structures.
Today Mr. Henderson started out the class by reviewing last nights scribe's blog. He went over all the plus points of it and went into further detail on how the blog reviewed what we did in class the previous day. He also talked about the 5 postulates. He then told us a little about what we were going to do today in class. Today in class our main goal was to learn about the atomic structure, atomic number, and the mass number.He then started to review page one in the packet. We went over most of it yesterday but we finished up today. We talked about how the Dalton theory of an atom was just a circle with the element name inside. There was no proposed structure to it. The JJ Thomson theory of an atom was that it looked like plum pudding. The Rutherford theory was that positive charges were concentrated in the nucleus which was in the center of the atom and then the electrons surrounded the empty space outside the nucleus. Bohr's theory of the atomic structure was that electrons are in orbit around the nucleus. Bohr's structure also only worked for hydrogen.The picture on the left shows these structures.





 of protons plus the number of electrons. It is the identifying property of what the element is. The mass number is the number of protons plus the number neutrons. The mass number is on top and the atomic number is on the bottom as showed in the picture. On the other side of the atomic symbol there would be either a +, -, or no sign there. This determines the charge of the electron and the over all change. It also determines whether it is a positive, negative, or neutral charge. The symbol and the subscript go together. We then continued to talk about isotopes. Isotopes are different types of atoms of the same chemical element, each having a different number of neutrons. They also differ in mass but never in atomic number. The number of protons is the same because that is what characterizes a chemical element. We took the element carbon as an example. On the periodic table when you see the element carbon, you see its atomic symbol, the number of electrons its has, and you see a number at the bottom called the super script. The super script is the average of the mass number of the atomic element. For carbon on the periodic table it shows 12.01. This means that the most abundant isotope of carbon has a mass of 12.01. Mr. H then explained the different between an Ion and an Atom. An Ion is a charged particle while an atom is a neutral particle.
of protons plus the number of electrons. It is the identifying property of what the element is. The mass number is the number of protons plus the number neutrons. The mass number is on top and the atomic number is on the bottom as showed in the picture. On the other side of the atomic symbol there would be either a +, -, or no sign there. This determines the charge of the electron and the over all change. It also determines whether it is a positive, negative, or neutral charge. The symbol and the subscript go together. We then continued to talk about isotopes. Isotopes are different types of atoms of the same chemical element, each having a different number of neutrons. They also differ in mass but never in atomic number. The number of protons is the same because that is what characterizes a chemical element. We took the element carbon as an example. On the periodic table when you see the element carbon, you see its atomic symbol, the number of electrons its has, and you see a number at the bottom called the super script. The super script is the average of the mass number of the atomic element. For carbon on the periodic table it shows 12.01. This means that the most abundant isotope of carbon has a mass of 12.01. Mr. H then explained the different between an Ion and an Atom. An Ion is a charged particle while an atom is a neutral particle.
Monday, September 27, 2010
Monday 27, September
On Pg. 15 in your chemistry Unit2 packet, there is a very general history concerning the atomic model starting with Dalton. However, Mr. Henderson told us that the first ever recorded theory concerning the atom dates all the way back to Ancient Greece and a philosopher by the name of Democritus. Democritus argued that he could chop a piece of papyrus into halves and halves and halves until he would reach some sort of building block that could not be broken down any further into a smaller or simpler form. He called these building blocks atomos and he believed they made up all matter. Democritus had no idea how accurate he actually was, but for nothing more than an educated guess, this was a remarkable theory. It is important to note that Democritus never actually tested this theory using science and therefore, he is regarded as a philosopher rather than a scientist. It was not until the late 1700's and early 1800's that the scientific revolution ushered in a new wave of interest in the field of atomic structure.
The next man to take a stab at an accurate atomic model was John Dalton. This scientist used the scientific method of developing a hypothesis and testing it to form a theory. He called these theories postulates, or basic rules. The five postulates that Dalton formed are as follows.
#1) All matter consists of atoms
#2) Atoms are indivisible (cannot be broken down any further)
#3) In a chemical change or reaction, atoms simply rearrange themselves but never turn into any other atoms. This is demonstrated in the picture below in which Hydrogen and Oxygen combine to form H20.

#3 (continued) Also, atoms never gain or lose mass in any way during any chemical or physical change.
#4) Compounds have a determinable amount of atoms and always have the same amount of each atom.
#5) Law of Constant Composition. All Compounds contain the same amount of each atom no matter where the compound is found or under what circumstances.
These 5 postulates are still seen today as a basic level in understanding the structure of atoms and compounds. Dalton never did create a finite model that he believed was what an atom might look like. He did however represent an atom as a circle with a symbol on it, such as H for Hydrogen.
Proceeding John Dalton, J.J. Thomson was at the forefront of the atomic model issue. J. J. Thomson believed that atoms did not only consist of alpha particles, but also possessed some other feature. By separating the alpha particles from a beam of light and firing this beam, known as a cathode ray, through a glass tube with positive or negative plates on either side Thomson was able to test this. After seeing the cathode ray bend towards the positive plate, Thomson was able to determine that the are other particles that make up an atom and that they are negatively charged. Thomson called these negatively charged particles electrons. This lead Thomson to relate his atomic model to the favorite desert of England at the time, earning it the name The Plum Pudding Atom. Thomson believed that atoms consisted of moving negatively charged electrons that are immersed in a sea of positively charged stationary particles. The model looked something like this picture, with the dots being electrons and the red material being the positively charged "sea".

For many years, A scientist by the name of Ernest Rutherford studied this model and debated it's accuracy. To test the Thomson models validity, Rutherford set up an experiment in which he took a thin (only a few hundred atoms thick) piece of gold foil and placed it in a container which had sensors on either side while shooting a stream of atoms in its direction. If Thomson's model was correct, the atoms should have gone through the gold foil with no problem and triggered only the sensors on the other side. What Rutherford found was that although most of the atoms did behave this way, 1 out of every 10/20,000 deflected off of the foil and reflected back at an acute angle. Rutherford was quoted saying "Its as if you shot 20,000 bullets at a tissue with most of them going through the tissue with no problem, but 1 out of every 20,00 of those bullets would reflect back at you!" Rutherford was amazed at this and determined that atoms were not full of positive particles, but were rather made up mostly of empty space with 99% of its mass concentrated in its nucleus, earning his model the name The Empty Space model. Rutherford still did not know how the electrons were distributed across the atom, leaving his model still somewhat inaccurate
Finally, one of Rutherford's colleagues, Niels Bohr, measured light energy to more accurately determine where the electrons would be relative to the nucleus of the atom. By measuring each layer of electrons of an atom and observing the light they emitted, Bohr was able to establish the general amount of electrons and their area relative to the nucleus. The downfall to Bohr's method was that once he began getting into the atoms with more electrons, such as Gold, his estimates were off by more than 20-40% at times. However, Niels Bohr was able to develop an atomic model, known as The Planetary Model.

Neils Bohr called it this because he thought that the electrons orbited the nucleus much like moons orbit a planet. This is the most accurate of all the depictions discussed so far.
To wrap up the class, Mr. Henderson gave us the answers to page 15 which are as follows:
1: b
2: d
3: c
4: a
5: c, b, a
We also drew sketches of what each scientist, (Dalton, Thomson, Rutherford, Bohr) believed
that atom looked like and those can be seen by asking me or any other student before or after class. Tomorrow, we will incorporate verbal explanations with these visuals models to describe each scientist views on concerning the structure of an atom.
Sunday, September 26, 2010
Friday, September 26
Friday, September 24, 2010
Thursday, September 23 - Unit 2
The unit involved a lot of learning - much of which was unrelated to course content yet nontheless important. Consider all you have learned that is not normally associated with chemistry:
- How to do a lab report
- How to remain safe in a laboratory
- How to find our course page at gbschemphys.com
- How to use ChemThink
- How to use WebAssign to do a reading sheet
- How to use WebAssign to complete a question/problem set
- How to use the Graphical Analysis software package
- How to use Delicious to make and share a bookmark
- How to use log in and create a blog post
Now that we have learned the Chemistry Basics and mastered the logistical items above, we will move a little quicker. The second unit pertains to Atoms, Molecules and Ions. We begin Friday with a laboratory.
Wednesday, September 22
Tuesday, September 21, 2010
Tuesday, September 21
 rate and imprecise throws, the other portraying accurate, but imprecise throws, and the last one portraying both accurate and precise throws. Here is a picture relating to this idea.
rate and imprecise throws, the other portraying accurate, but imprecise throws, and the last one portraying both accurate and precise throws. Here is a picture relating to this idea. 
Monday, September 20
 |
| This is a picture of my lab group’s data table from lab #8 |
Saturday, September 18, 2010
Friday, September 17



Thursday, September 16, 2010
Thursday, September 16
NOTES FROM CLASS DISCUSSION:
The big idea was that every substance has a unique set of identifying properties; these properties distinguish the substance from other substances. For example: Water is a colorless substance that boils at 100 degrees C, freezes at 0 degrees C, has a density of 1.0 g/mL and a heat capacity of 4.18 J/gC.
· Intensive vs. Extensive Properties
o Intensive- identifying does not depend on amount
o Extensive- (usually volume and mass) depends on amount
· Chemical Properties: describes how a substance reacts (or not) with other substances
· Physical Properties: describes a substance apart from how it reacts
· Three common physical properties: density, solubility, and color
· DENSITY
o A measure of how tightly that mass is packed into a given volume of space
o Density= mass/volume
o Units: g/cm^3, g/mL, kg/L, kg/mL
After Mr. H explained density, he went through the answers to the 1.3 reading sheet:
1. .A
2. .A. PP, B. CP, C. PP, D. CP
3. .FALSE- The density of a material is specific to that material and not dependent on the amount.
4. .A
5. .D
6. B
7. .C
8. .A
Mr. H noticed that many people didn’t understand how to do number 4, which involves knowledge of significant digits. If you still don’t understand or would like to practice it more then you should go to this website or see packet page 6: http://www.physics.uoguelph.ca/tutorials/sig_fig/SIG_dig.htm
After we did our review and notes, Mr. H explained Lab MM7. The lab was called Dense Cents Lab and the question was “What is the density value of pre- and post-1982 pennies? How do their density values compare? How can the difference be explained?” The purpose was to use a plot of mass vs. volume to determine and compare the density value of pre- and post-1982 pennies and to explain the difference between their densities. First each group separated the pennies into two groups, one with pre-82 and one with post-82 pennies.

Then we measured out 50 mL of water into a graduated cylinder. This was going to be used to find the volume of the pennies, using displacement. Displacement is where you add the objects to the water and difference between the original volume and the new volume is the volume of the object. We massed the pennies in groups of 5 up to 30. After massing each group of 5 we then put them in the graduated cylinder to find the volume. We then divided the volume from the mass of each set to find the density.


Based on the data that we collected, the pre-82 pennies seem to have a higher density. The pre-82 pennies had a total density of 50.095 and the post-82 pennies had a density of 53.545.

Wednesday, September 15, 2010
Wednesday, September 15
http://www.dropshots.com/chemistryclassroom
While showing the class Katie's blog, Mr. M noticed that Katie had embedded an link with excellent practice into her blog. This reminded him of telling us about the Delicious assignment that we have due on Friday, September 17. He said that Katie's link was a perfect example of what we could use for our assignment. He showed us how to log into the database and how to tag and fill out the form for our links. In order to log into this website, we need a Yahoo account. Mr. H told us that it would be useful to have a Yahoo, Google, or Facebook account because those major corporations would end up taking over many of the smaller companies. Thank you for those wonderful words of wisdom, Mr. H! More thorough directions for Delicious can be found on the hand out we received in class on Monday or on the GBS Chem-Phys website:
http://gbschemphys.com/honchem/index.html
After our tech tutorial, Mr. Henderson asked us to take out our unit packets and turn to page 9. Before beginning the worksheet, he quickly reminded us of the differences between a physical (describing the substance without describing its interaction with other substances) and chemical (describing the substance by how it interacts with other substances) properties. To give us a better idea, he told us he would show us an example of a chemical property of magnesium. He showed us the sample of magnesium - a shiny metal - and turned on the methane flow. He lit the burner (from the bottom up, of course) and began to burn the magnesium. Mr. H told us that magnesium reacted very strongly with oxygen to make magnesium oxide and that we should be prepared to see a very bright light, indicating the reaction. Sure enough, when the magnesium heated up enough, we saw a very bright light. Our experience was similar to this video from Youtube:
http://www.youtube.com/watch?v=Q_LU1EASadU
Mr. H then pulled up an overhead of 4 out of the 5 pieces of evidence of a chemical change. They are: bubbling & production of gas, formation of a solid, heat or light, color changes, or temperature changes. The difference between a heat/light change and a temperature change is that the temperature change is more drastic and long term.
As soon as all the explanation was finished, we began page 9 and the "Verbal Description of Change" section. We attempted to do the section individually and went over the activity together and the answers are as followed: C, P, C, C, C, P, P, C. We had to be on the lookout for the chemical changes we learned about in each of the scenarios order to determine the type of change.
Next, we determined the "Symbolic Description of Change" as a class. If the element remained the same, it was a physical change. If the before and after differed from each other, it was a chemical change. The answers are as followed: P, C, C, P.
To finish the page, we flipped it over and determined the "Visual Depiction of Change at Microscopic Level". We had to be on the lookout for changes in the shape and arrangement of the atoms and molecules. The answers are as followed: C, P, C, P.
Abruptly, Mr. H got up and directed us to the back of the room and under the fume hood. He asked us for two pennies. He took our a large bottle of nitric acid and explained to us what a hazardous chemical it was and how we had to be careful when handling it. Mr. H was going to demo the reaction between copper and this nitric acid. He poured the acid into a flask and diluted it with some water. He dropped one of the pennies in and told us to observe. Meanwhile, he took another flask and filled it with pure nitric acid. He dropped the copper penny into the substance and told us to observe and compare it to the other flask. Immediately, we saw a change of color. The acid went from clear to a gradient green-red. Bright orange gas began spewing out of the top of the beaker. It bubbled and displayed a clear chemical change. The diluted beaker was showing the same changes (minus the color change and significantly less bubbling and gas) at a much slower rate. Mr. H ask Katie to touch the beakers carefully. She told the class they were both very warm, indicating a temperature change. It was very interesting to see the copper penny dissolving and reacting with the acid. The flask with the pure nitric acid and penny looked similar to this photo:
 We concluded the class by returning to our seats. Mr. Henderson quickly read us a story about a man whose experience with nitric acid inspired him to keep experimenting with it. With little time left, we were instructed to turn to page 11 and complete the "Verbal Description of Change" and determine the Physical and Chemical properties. The answers are as followed: P, C, C, P, C, P, C, C, P.
We concluded the class by returning to our seats. Mr. Henderson quickly read us a story about a man whose experience with nitric acid inspired him to keep experimenting with it. With little time left, we were instructed to turn to page 11 and complete the "Verbal Description of Change" and determine the Physical and Chemical properties. The answers are as followed: P, C, C, P, C, P, C, C, P.
Just as the bell was about to ring, Mr. H handed out the quizzes from last week and told us to check our answers and use it as a guide. He told us that tomorrow we could expect a lab about density and that our homework was finishing the 1.2 Webassign and our Delicious assignment.
Friday September 10, 2010
Mr. H then had everyone take out their chemistry packets and he went over the question on the bottom of page 6, which was about listing significant numbers when adding, subracting, multiplying, and dividing. He went over significant numbers again and how many to include when adding, subtracting, multiplying, and dividing. The rules are as followed when adding/subrtracting or multiplying/dividing:
Addition/Subraction: The number of decimal places in the result is equal to the number of decimal places in the quantity with the least certainty (i.e., least number of decimal places).
Multiplication/Division: The number of significant figures in the result is the same as that of the quantity with the least number of significant figures.
Mr. H then worked with the class on the first two practice problems to clarify what the rules were saying. He then had us work on the rest of the practice problems by ourselves. After a few minutes of working Mr. H revealed the answers to the class, so everyone was able to check their answers. He answered a few questions from students and then he went to our class's chem blog.
Mr. H went over Hannah's and Neil's blog posts and then gave a few tips on how to make both blogs a bit better for next time.
Mr. H then had the class open up their chemistry packets to the last page to look over today's lab, which was MM6 Conservation of Mass Lab. As the class wrote down the purpose, he explained what we were going to do in today's lab and what to expect. He told the class that since we were going to be dealing with liquids and chemical reactions, that we should wear our safety glasses. He explained to us that we were to find the mass of a flask before the chemical reaction and after, and then compare the differences.
 |
| Click to view video. |
Tuesday, September 14, 2010
Tuesday, September 14
 If you are trying to convert the numbers from scientific notation to the standard decimal notation, you have to move the decimal place and then fill in zeros. If there is a negative exponent, you will move the decimal point to the left ____ spaces. If there is a positive exponent, you will move the decimal point to the right ___ spaces. Like I said before, significant figures are still in effect for these problems.
If you are trying to convert the numbers from scientific notation to the standard decimal notation, you have to move the decimal place and then fill in zeros. If there is a negative exponent, you will move the decimal point to the left ____ spaces. If there is a positive exponent, you will move the decimal point to the right ___ spaces. Like I said before, significant figures are still in effect for these problems. The last section (3) on page 8 in the unit packet is practice using scientific notation on a calculator. When entering the operations on the calculater, you have to make sure to include all parentheses in order for the answer to come out correct. There is a special "EE" button on the CAS calculators especially for scientific notation. After getting an answer on the calculator, you were then to put those answers back into scientific notation, but more simplified than the original.
The last section (3) on page 8 in the unit packet is practice using scientific notation on a calculator. When entering the operations on the calculater, you have to make sure to include all parentheses in order for the answer to come out correct. There is a special "EE" button on the CAS calculators especially for scientific notation. After getting an answer on the calculator, you were then to put those answers back into scientific notation, but more simplified than the original.  The last thing we talked about in class was chemical and physical properties. A chemical property is a property of a substance which describes how the substance interacts (reacts) with another substance. A physical property is a property of a substance which describes the substance without describing its interaction with other substances. This was followed up by a demo lab where Mr. Henderson heated a colorless test tube over a methane burner. The test tube then turned a purplish color and took solid iodine and changed it to a gaseous iodine. This was used during the process of sublimation which includes freezing, melting, boiling, etc.
The last thing we talked about in class was chemical and physical properties. A chemical property is a property of a substance which describes how the substance interacts (reacts) with another substance. A physical property is a property of a substance which describes the substance without describing its interaction with other substances. This was followed up by a demo lab where Mr. Henderson heated a colorless test tube over a methane burner. The test tube then turned a purplish color and took solid iodine and changed it to a gaseous iodine. This was used during the process of sublimation which includes freezing, melting, boiling, etc. 











