
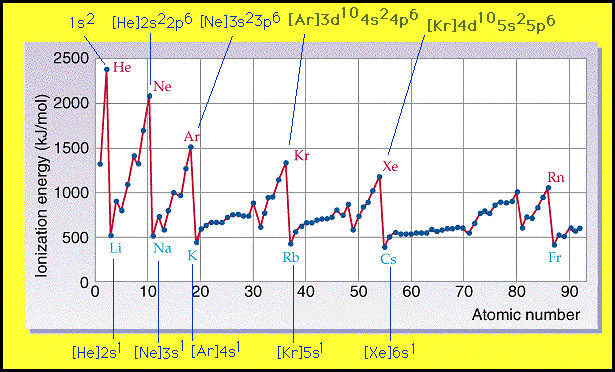
Today’s class was very informative. We started out by first going over Will’s blog from yesterday. Mr. H kept saying what a great job Will had done, and how he really liked one of Will’s graphics, which I have to agree with. Since this graphic is also relevant and helpful with my post, I will also put it up.
We then started doing some practice problems in our Lab Notebooks, problems that are very similar to one of the two webassigns due for homework tomorrow.
Before we go over some problems, just remember these key things:
• All orbitals in a sublevel must have one electron in them before they can have two.
• To write an Abbreviated Electron Configuration, you first go to the previous rows noble gas to use as the starting point
• In the d-block, the rows start with 3d, not 4d as one would suspect
• When dealing with elements that go into the 5d or 6d orbital, ADD THE F-BLOCK ORBITALS IN IT!
Here are some examples of doing Abbreviated Electron Configurations:
Aluminum: [Ne] 3s2 3p1 explanation: Ne is the noble gas in the row above Al. Al fills the 3s orbital, which can hold up to 2 electrons which is why the superscript is 2. Then, Al fills up only 1 electron in the 3p orbital.
Lead: [Xe] 6s2 4f14 5d10 6p2 explanation: Xe is the noble gas in the row above Pb. Pb fills up the 6s orbital, which can hold up to 2 electrons, which is why the superscript is 2. Next, Pb fills up all 14 electrons of the 4f level, which is why the superscript for that is 14. Then, it also will fill up the 5d row. Finally, it fills up only 2 electrons of the 6p level.
Tl: [Xe] 6s2 4f14 5d10 6p1 explanation: Xe is the noble gas in the row aboveTl. Tl fills up the 6s orbital, which can hold up to 2 electrons, which is why the superscript is 2. Next, Tl fills up all 14 electrons of the 4f level, which is why the superscript for that is 14. Then, it also will fill up the 5d row. Finally, it fills up only 1 electron of the 6p level.
*if you want to make sure you did it right, you can add up all the super scripts in the abbreviated electron configuration and add it to the atomic number of the noble gas in the brackets.
Next we went on to IONS
Ions are an atom or molecule in which the total number of electrons is not equal to the total number of protons, which we learned earlier this year. Ions are in the excited state.
Take, for example, a sulfur ion (S2-). To write a abbreviated electron configuration for it all you have to do it write the abbreviated electron configuration for Argon, because they have the same number of electrons.
Ground state vs. Excited state
Ground State for chlorine:[Ne] 3s2 3p5
Excited State for chlorine: [Ne] 3s1 3p6 OR [Ne] 3s2 3p4 4s1
Take note that each of the three configurations has the same amount of electrons, just on different levels. The electrons will eventually give off light and jump down the levels to its ground state.
We ended today in the computer lab working on the webassign due tomorrow.



 Next, we reviewed #7 on packet page 8 which is similar to the quantum mechanics 3 web assign number 2. The question states: An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. From that you were to determine the atomic number, total number of electrons, mass number, and total number of s/p/d electrons. To figure this question out you count the number of electrons stated in the problem. In this case there would be 25 electrons (don't forget to count the electrons in n=1 and n=2)/ Since it has a 2+ charge, the element would have the atomic number of 27. You could then use this information to answer the remaining questions.
Next, we reviewed #7 on packet page 8 which is similar to the quantum mechanics 3 web assign number 2. The question states: An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. From that you were to determine the atomic number, total number of electrons, mass number, and total number of s/p/d electrons. To figure this question out you count the number of electrons stated in the problem. In this case there would be 25 electrons (don't forget to count the electrons in n=1 and n=2)/ Since it has a 2+ charge, the element would have the atomic number of 27. You could then use this information to answer the remaining questions.









