Next, we went over the periodic trends which is similar to what we did in the computer lab yesterday. Graphs, diagrams, and explanations can be found on packet page 9. There are three topics discussed on packet page 9: size of atom, ionization energy, and electronegativity.
There are two noticable trends that can be found looking at the size of the atom. As you go down a column, the size of the atom increases. As you go across a period, the size decreases.
There is a diagram provided below to be used as a visual to show these two trends.

When looking at a graph of ionization energy the trends seem to be the opposite to the size of the atom. (note: ionization energy is the amount of energy required to remove an electron from an atom). As you go down a column, the ionization energy decreases. As you go across a period, the ionization energy increases. Below is a graphic demonstrating the trends for ionization energy.
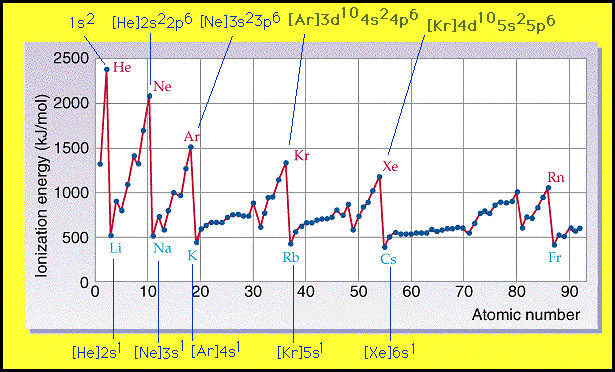
Both of these two periodic trends discussed are discussed and mentioned in lab AS2.
The last graphic we looked at was electronegativity. (note: electronegativity relfects the tendency of an atom to attract the electrons in a bond with an atom of another element--noble gases aren't listed for this graph because they do not usually form bonds) The trends noticed were when you went down a column, the electronegativity decreases. Also, when you went across a period, the electronegativity inceases. Below is a graphic on the trends of electronegativity in elements.
 Next, we reviewed #7 on packet page 8 which is similar to the quantum mechanics 3 web assign number 2. The question states: An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. From that you were to determine the atomic number, total number of electrons, mass number, and total number of s/p/d electrons. To figure this question out you count the number of electrons stated in the problem. In this case there would be 25 electrons (don't forget to count the electrons in n=1 and n=2)/ Since it has a 2+ charge, the element would have the atomic number of 27. You could then use this information to answer the remaining questions.
Next, we reviewed #7 on packet page 8 which is similar to the quantum mechanics 3 web assign number 2. The question states: An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. From that you were to determine the atomic number, total number of electrons, mass number, and total number of s/p/d electrons. To figure this question out you count the number of electrons stated in the problem. In this case there would be 25 electrons (don't forget to count the electrons in n=1 and n=2)/ Since it has a 2+ charge, the element would have the atomic number of 27. You could then use this information to answer the remaining questions.After that problem we worked on the Web Assign using netbooks.
The answer to number 2 was eventually given to us, after having complications with answers.
#2:
a) 24
b)6
c)12
d)0
e)26
f) 1s2 2s2 2p6 3s2 3p6 4s2 3d4
HOMEWORK:
--delicious assignment
--web assign
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.