

This is a cooperative effort of our period 3 class to document what occurs in class on a daily basis. This is "our book", written by us, for us (and for whomever else stops by). Each day, one student is the "scribe". Before the next class, that student "adds a post" in which he/she explains what happened in class. Concepts must be explained and documented. Examples, diagrams, graphs, scanned worksheets, links, photos or videos (taken with a camera or cell phone) can be included.


 After the problem, we switched to a second lab that was an extension of the lab that we had done the previous day. It was focused around making specific calculations with the information that we are given. Since this lab was very dangerous, Mr. H did it himself as the class watched. Mr. H performed a reaction where before the reaction there were 91.46g of Sucrose with the beaker and after there were 67.28g of of Carbon with the beaker. The third piece of data that we had was the mass of the beaker which was 50.66g. The three calculations that we had to make where the mass of Sucrose, Carbon and H20. The mass of Sucrose was found by taking the subtracting the mass of the sucrose with the beaker and subtracting the mass of the beaker. There were 40.80 g. The mass of carbon was the mass of carbon after the reaction which was 67.28g was subtracted by the mass of the beaker which meant that there were 16.62g of Carbon in Sucrose. The mass of H20 was the mass of sucrose subtracted by the mass of carbon. The mass of H20 was 24.18g. Then, the percents of Carbon and H20 in sucrose was found. The equations follows.
After the problem, we switched to a second lab that was an extension of the lab that we had done the previous day. It was focused around making specific calculations with the information that we are given. Since this lab was very dangerous, Mr. H did it himself as the class watched. Mr. H performed a reaction where before the reaction there were 91.46g of Sucrose with the beaker and after there were 67.28g of of Carbon with the beaker. The third piece of data that we had was the mass of the beaker which was 50.66g. The three calculations that we had to make where the mass of Sucrose, Carbon and H20. The mass of Sucrose was found by taking the subtracting the mass of the sucrose with the beaker and subtracting the mass of the beaker. There were 40.80 g. The mass of carbon was the mass of carbon after the reaction which was 67.28g was subtracted by the mass of the beaker which meant that there were 16.62g of Carbon in Sucrose. The mass of H20 was the mass of sucrose subtracted by the mass of carbon. The mass of H20 was 24.18g. Then, the percents of Carbon and H20 in sucrose was found. The equations follows.

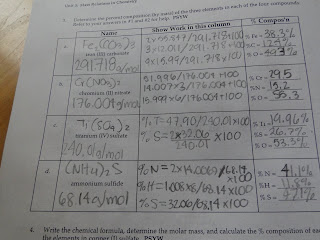 After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.
After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.


We then started to complete page 10. Part one was identified as ionic compounds and we therefore knew we were dealing with metals. The answers are as followed:
a) Magnessium iodide
b) Sodium sulfide
c) Calcium bromide
d) (Typo possibly?)
Next, we identified part two as ionic compounds with multi-valent cations (transitional metals). The answers are as followed:
a) Copper(II) chloride
b) Cobalt(II) sulfide
c) Manganese(III) chloride
d) Copper(I) sulfide
For polyatomic ions (part 3), we only went over b. The answer is Ammonium sulfide.
For part 4, the reverse was to be done. The answer to e was (NH4)2S and to f was (NH4)3PO4.
Mr. H then reminded us the "training wheels" when formulating compounds. He told us to first identify the charges of the ions and the properties of balancing charges and then begin to put together the formula.
Subsequently, we were re-taught how to put together the formulas of molecular compounds. Greek prefixes must be used to indicate the number of atoms of each element in the compound. The only exception to this rule is cases in which there is only one atom of the first element. Further practice can be found on page 11 in the packet.
Our review ended with a quick lesson on putting together the formulas of acids. They generally have the chemical formula HX where X is the nonmetal. Their names take the form of hydro(anion root)ic acid.
( i.e. hydrochloric acid - HCl)
The general formula of an acid and how to create it can be found in Daria's blog before me although remember:
-ate --> -ic
-ite --> -ous
Before passing out our quizzes, Mr. H showed us a brief video on experimenting with the reactions between water and alkali metals. The video can be accessed here:
http://www.youtube.com/watch?v=eCk0lYB_8c0
After this, Mr. H handed out Periodic tables and our pop quizzes and....
THE END!
Y






 After we were done with page seven, we moved to page eight. On this page, we had to name ionic compounds, but they were different than the ones on page seven. They had roman numerals next to their names. The roman numerals represented the charge of the metals. Also, all the metals were transition metals and Pb+Sn. All these metals could posses different types of charges. For example, iron could have 2, 3, or 5 charges and rarely 4 or 6 charges. Afterwards, we were given problems six and seven to complete. Problem six was similar to problem five on page seven and problem seven was similar to problem six on page seven. The answers to problem six were:
After we were done with page seven, we moved to page eight. On this page, we had to name ionic compounds, but they were different than the ones on page seven. They had roman numerals next to their names. The roman numerals represented the charge of the metals. Also, all the metals were transition metals and Pb+Sn. All these metals could posses different types of charges. For example, iron could have 2, 3, or 5 charges and rarely 4 or 6 charges. Afterwards, we were given problems six and seven to complete. Problem six was similar to problem five on page seven and problem seven was similar to problem six on page seven. The answers to problem six were:FeBr3- iron (III) bromide
NiS- nickel (II) sulfide
CoCl2- cobolt (II) chloride
FeBr2- iron (II) bromide
AuCl3- gold (III) chloride
SnF2- tin (II) fluoride
The answers to problem seven were:
copper (I) chloride- CuCl iron (III)oxide- Fe2O3
tin (IV) fluoride- SnF4 iron (II)sulfide- FeS
After answering the problems on page eight, class ended. For homework, we were given a choice to do some of the work at home or to work on it at the computer lab tomorrow. If you require a more detailed explanation on how to name ionic compounds, I recommend you check out this website.
http://chemistry.about.com/od/nomenclature/a/nomenclature-ionic-compounds.htm
 After we finished going over the blog Mr. Henderson told us to open up our packets to page five. This page was going to help us learn about ions. Ions are negatively or positively charged particles. In other words anything with a charge is considered an ion. There are two types of ions: Cantions, which are positive and have more protons then elections, and Anions, which are negative and have more elections then protons. Mr. Henderson gave us a great way of remembering which one is which, for Anion he told us, A N Ions, which stands for A= a, N=negative, Ions=ion, so a negative ion! Also, all metals tend to form positive ions and all non-metals tend to form negative ions.
After we finished going over the blog Mr. Henderson told us to open up our packets to page five. This page was going to help us learn about ions. Ions are negatively or positively charged particles. In other words anything with a charge is considered an ion. There are two types of ions: Cantions, which are positive and have more protons then elections, and Anions, which are negative and have more elections then protons. Mr. Henderson gave us a great way of remembering which one is which, for Anion he told us, A N Ions, which stands for A= a, N=negative, Ions=ion, so a negative ion! Also, all metals tend to form positive ions and all non-metals tend to form negative ions. |
| Mr. Henderson showed us what sodium looks like when it comes out of the container. It was a little rusty looking and defiantly not shiny. |
 |
| Mr. Henderson demonstrates how sodium “cuts like butter”. |
 |
| After cutting the sodium, it is REALLY shiny and nice looking. |

 e following: (To the left)
e following: (To the left)

Friday October 1, 2010
Today we started out our day as we always do, we went over the previous nights’ blog and recapped all the important ideas we learned yesterday. We also got a quick preview of the sodium experiment we were going to do during today’s class period.
After we finished going over the blog Mr. Henderson told us to open up our packets to page five. This page was going to help us learn about ions. Ions are negatively or positively charged particles. In other words anything with a charge is considered an ion. There are two types of ions: Cantions, which are positive and have more protons then elections, and Anions, which are negative and have more elections then protons. Mr. Henderson gave us a great way of remembering which one is which, for Anion he told us, A N Ions, which stands for A= a, N=negative, Ions=ion, so a negative ion! Also, all metals tend to form positive ions and all non-metals tend to form negative ions.
In the worksheet there was some hard questions with multiple answers that seemed right but really weren’t. For example, in numbers 3 and 4 we are given 6 answers to choose from, but Mr. Henderson made it easy. He gave us an easy way to make some answers obviously wrong. He said that, splitting nucleuses causes atomic bombs so obviously that’s not what an ion is. But, splitting elections is. So in numbers 3 and 4, four of the answers are wrong at first sight since they say losing a proton, gaining a proton etc. So were left with two answers for each question which makes it twice as easy to pick the right one.
For number five Mr. Henderson told us that “Atoms of most elements want to be like HeNeArKrXeRn.” Therefore when looking for the charges of atoms, all you have to do is either subtract the atomic number to make one of the atoms in group 18 or add to the atomic number to make one. This makes making ions so much easier.
For today we skipped number six. But we did half of number seven and left the last two for Monday. Number seven is an easy practice question which we have done on page two and gotten a lot of practice on it. But this time there’s a twist, you add ions. When writing an ion you write the charge (+,-) then the number, for example the first element we do is magnesium-24 ion. The isotopic symbol is 1224Mg+2.
After doing page 5 Mr. Henderson showed us by far one of the coolest demos we have ever done. It was dropping sodium in water, and it made for a great show. The sodium, which is highly reactive in water since it is in group one, started fizzing and going in a circle. It was really cool.

In the first picture Mr. Henderson showed us what sodium looks like when it comes out of the container. It was a little rusty looking and defiantly not shiny.

In the second picture Mr. Henderson demonstrates how sodium “cuts like butter”.

In the third picture after cutting the sodium, it is REALLY shiny and nice looking.
Here is a link to the video so you can see exactly what happened, remember the password to sign in is gbs. http://www.dropshots.com/chemistryclassroom#date/2010-09-30/21:41:02
After these fun activities we finished the class with a couple videos and Mr. Henderson giving us a preview of the homework and of what we’re going to do on Monday.
The first video we showed was http://www.youtube.com/watch?v=GDMUb5mQsjo. That video showed a not so exciting Caesium in water video from the funny haired guy. Since the reaction was not so exciting the whole class decided to watch a better reaction, which was http://www.youtube.com/user/periodicvideos#p/search/6/5aD6HwUE2c0. That gave off a VERY big reaction and was sufficient for our curiosity.
After the videos Mr. Henderson told us we are officially done with section four and sections 5 and 6 are going to take a week or a little more after that. During next week, on Tuesday and Wednesday we are going to be in the computer lab. We are going to be working on lab 4, which Mr. Henderson is going to explain, and on web assigns that we have not completed yet.
In lab number four we have a lot of data to get; since we are done in the back of the room with this lab Mr. Henderson was nice enough to record the rest of the data on t
he homepage of his chemistry honors website. It is located under the title “Help is Hear!” That recording will help you with the rest of the lab, but we will be doing that on Wednesday and Thursday.
Finally Mr. Henderson finished the exciting day in the chemistry room. The homework for next time was a web assign due Monday on chapter 2.4, and an ongoing assignment to complete your lab notebook, which is due Friday.
If you need any help on ions I recommend these two websites. In the first site they help with naming the ions, which you will learn in chapters 2.
5 and 2.6 but if you would like to get a head start this site is a good preview. In the second website there is a magnificent review of how to figure out the charges of ions and how to make compounds neutral. I defiantly recommend the second website for a review for the test.
http://www.mpcfaculty.net/mark_bishop/ionic_nomenclature_help.htm
http://www.wisc-online.com/objects/ViewObject.aspx?ID=gch2104