Today’s class began with a celebration of mole day! We then looked through last night’s blog post. After, we worked on pages 11 and 12 of our unit packet, which is about molar mass and percent composition. These are the steps to find figure that out,
1) Write out the formula for the ionic compound
2) Figure out how many atoms there are for each of the elements
3) Multiply the number of atoms by the mass number for each of the elements
4) Add that all together to find the total molar mass (g/mol)
5) To find the percent composition, divide the molar mass for each atom by the total molar mass 6) Once the answer is found, add 100, so the number is a percent
7) Do the same for each of the atoms (the percents should be equal to 100)
For example, iron (III) carbonate has a formular of Fe2(CO3)3. This contains 2 atoms of Fe, 3 atoms of C, and 9 atoms of O. The mass number of iron is 55.857, which is multiplied by 2 because there's 2 atoms of it. Then, add 3x12.011 (Carbon) and 9x15.99 (Oxygen). This number is 291.72 g/mol, which is the molar mass. To find the percent composition, 55.847x2 divided by 291.78 and add 100, which is 38.3 percent. The percentages for Carbon and Oxygen are 12.4% and 49.3%. Mr H also taught us a shortcut to find the 3 percent: add the 2 previous percentages and subtract that from 100.
For extra help on percent compositions, http://www.ausetute.com.au/percentc.html gives a thorough and detailed explanation and provides examples.
The answers for page 12, the answers should look like this:
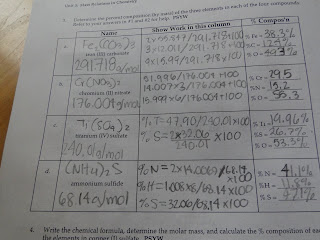 After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.
After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.
1) Write out the formula for the ionic compound
2) Figure out how many atoms there are for each of the elements
3) Multiply the number of atoms by the mass number for each of the elements
4) Add that all together to find the total molar mass (g/mol)
5) To find the percent composition, divide the molar mass for each atom by the total molar mass 6) Once the answer is found, add 100, so the number is a percent
7) Do the same for each of the atoms (the percents should be equal to 100)
For example, iron (III) carbonate has a formular of Fe2(CO3)3. This contains 2 atoms of Fe, 3 atoms of C, and 9 atoms of O. The mass number of iron is 55.857, which is multiplied by 2 because there's 2 atoms of it. Then, add 3x12.011 (Carbon) and 9x15.99 (Oxygen). This number is 291.72 g/mol, which is the molar mass. To find the percent composition, 55.847x2 divided by 291.78 and add 100, which is 38.3 percent. The percentages for Carbon and Oxygen are 12.4% and 49.3%. Mr H also taught us a shortcut to find the 3 percent: add the 2 previous percentages and subtract that from 100.
For extra help on percent compositions, http://www.ausetute.com.au/percentc.html gives a thorough and detailed explanation and provides examples.
The answers for page 12, the answers should look like this:
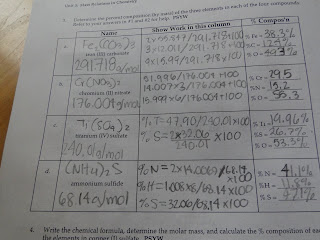 After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.
After doing pages 11 and 12, we went over yesterday’s lab and turned in our lab notebooks to be graded. Our lab notebooks included lab MR1 (the H20 Challenge Lab) and MR2 (Chew on This! Lab). To finish class, we took a short quiz over 3.1 and 3.2, which included many conversions.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.