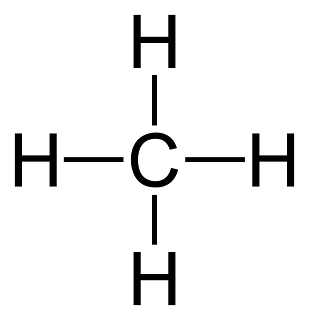
What is this, you ask? It's a Lewis structure of a methane molecule, or CH4. As you can see, carbon is the central atom, while the hydrogens attach themselves to it. Every atom is happy because each hydrogen gets two electrons, and the carbon gets the eight it requires. But what does this have to do with anything? Simply put, this explains how many electrons each atom in a molecule should get. This also explains why this molecule is non-polar, even though it has polar bonds. Finally, this dot structure reiterates the fact that methane would be composed in a tetrahedral shape, much like this:

We started class today with taking a closer analysis at pages 11-12 in our packets. Not to mention reviewing Lewis structures, we studied a new aspect of looking at molecules: we learned about the bonding type molecules have. All the possible bonding types go as follows:
sp --------------------> AX2
sp2 --------------------> AX3
sp3 --------------------> AX4
sp3d --------------------> AX5
sp3d2 --------------------> AX6
"But I'm confused, Dmitriy. What is it I see above?" Well, I'll tell you what. Those six values are what the layman would call "Hybridization types". The A represents the central atom, in methane's case the carbon. The X represents the surrounding atoms, the hydrogens. The numerical digit would represent how many surrounding atoms there are. As you already know, there may or may not be an E, which would represent unbonded electron pairs.
In short, these hybridization types show all the AX's and which spd configuration they would have.
Next, we introduced intermolecular as well as intramolecular forces on molecules.
"But I'm confused, Dmitriy."
Intermolecular - happening between different molecules, as in molecules acting on each other
Intramolecular - happening within an individual molecule, as in a molecule acting on itself
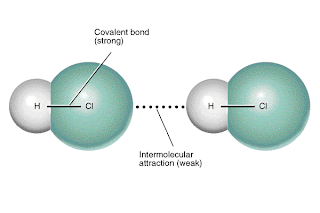
This is a weak intermolecular attraction. It is occuring between two HCl molecules. "But why is it weak?" Because hydrogen is not bonded with nitrogen, oxygen, or iodine. However, this diagram is very effective at conveying its message because it shows not only intermolecular forces, but intramolecular forces, as well. As you can tell, although the intermolecular forces are weak, the intramolecular forces (covalent bonds) are quite strong.
Furthermore, we discussed London dispersion forces as well as dipole attractions.
London dispersion:
1. temporary, short-lived
2. opposites attract (aka electrostatic)
3. greatest for larger molecules
Dipole-dipole:
1. longer-lived
2. electrostatic (same as above)
3. dipoles align selves
4. NOT experiences by non-polar substances
However, I do understand a picture is worth a thousand words. So I'll give you two thousand. Here are images of both these attractions to demonstrate the validity of the two arguments.
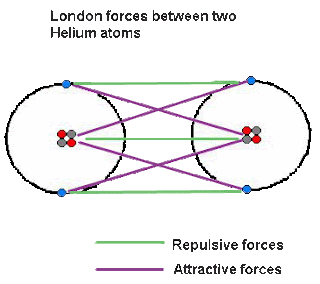

To summarize and conclude, we solidified our knowledge of chapter 9 to be better prepared for the TEST ON FRIDAY
Furthermore, we have instructions to finish our labs. Don't forget to look at the review and do the review WebAssigns!
Here's some chemistry jokes for you:
What do chemists call an iron ring?
A ferrous wheel
Why do professors like to teach about ammonia?
It's really basic stuff
What's the name of 007's Arctic cousin?
Polar bond
Problem, Will?
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.