We began with number 11. It was exactly like yesterday's problems. Step by step: first, you find the ratio of the products to reactants, like always. You plug in the information you have, but we only get the mole values. So, we convert to molarity. That is found by dividing moles by the liters given. Anyways, you plug in the molarity and then solve for x. Simple as that. The answer to number 11 is .00114 for NH3.
Following up with that question, we turned our attention to numbers 13 and 14. The equation is the reciprocal of number 11's equation, so you find the reciprocal of the answer, too. From there, you can figure out the temperature, which is 76.9. Then, Mr. H had us try and figure out number 14. He asked Konstantine and Daniel if the answer to number 14 is the doubled or squ
ared for .0113 or doubled/squared for 76.9. They both said that they think it is not doubled or squared, but kept the same. However, Neil, using his debate skills, countered saying that 76.9 is squared because if you do the ratio for the equation on number 14, it ends up that if you square one side, you have to square the other to keep it equal.
After their debate or whatever, we took the quiz, which was 2 questions. Mr. H said that if we found that easy, we are in good shape.
Finally, we turned to page 15. For number 1, the answer is C. Straight from the packet, for such a situation with a K value greater than 1, the system is described as having an equilibrium which favors the products or lies to the right. So pretty much,
the Kp is a ratio, so the products have to be greater than the reactants if Kp is going to be greater than 1.
When Kp is equal to the ratio, then it is at equilibrium. We learned that Q represents the ratio between products and reactants. When Q is greater than K, there are too much products. If Q is less than K, there is too much reactants. K wants to reach equilibrium, so this is very bad.
Solving is just the same. You find the ratio (products over reactants), raise the power to
the number of the coefficient, plug in the numbers given, and see if it is equal to K (which is given). Like I said before, if Q is greater, then there is too much products (numerator). If Q is less, there is too much reactants (denominator).
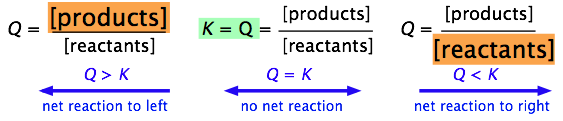
We ended with jokes from Mr. H's book. :)
There's a reading assignment due tomorrow so do it!
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.