


This is a cooperative effort of our period 3 class to document what occurs in class on a daily basis. This is "our book", written by us, for us (and for whomever else stops by). Each day, one student is the "scribe". Before the next class, that student "adds a post" in which he/she explains what happened in class. Concepts must be explained and documented. Examples, diagrams, graphs, scanned worksheets, links, photos or videos (taken with a camera or cell phone) can be included.



NOTES FROM CLASS DISCUSSION:
The big idea was that every substance has a unique set of identifying properties; these properties distinguish the substance from other substances. For example: Water is a colorless substance that boils at 100 degrees C, freezes at 0 degrees C, has a density of 1.0 g/mL and a heat capacity of 4.18 J/gC.
· Intensive vs. Extensive Properties
o Intensive- identifying does not depend on amount
o Extensive- (usually volume and mass) depends on amount
· Chemical Properties: describes how a substance reacts (or not) with other substances
· Physical Properties: describes a substance apart from how it reacts
· Three common physical properties: density, solubility, and color
· DENSITY
o A measure of how tightly that mass is packed into a given volume of space
o Density= mass/volume
o Units: g/cm^3, g/mL, kg/L, kg/mL
After Mr. H explained density, he went through the answers to the 1.3 reading sheet:
1. .A
2. .A. PP, B. CP, C. PP, D. CP
3. .FALSE- The density of a material is specific to that material and not dependent on the amount.
4. .A
5. .D
6. B
7. .C
8. .A
Mr. H noticed that many people didn’t understand how to do number 4, which involves knowledge of significant digits. If you still don’t understand or would like to practice it more then you should go to this website or see packet page 6: http://www.physics.uoguelph.ca/tutorials/sig_fig/SIG_dig.htm
After we did our review and notes, Mr. H explained Lab MM7. The lab was called Dense Cents Lab and the question was “What is the density value of pre- and post-1982 pennies? How do their density values compare? How can the difference be explained?” The purpose was to use a plot of mass vs. volume to determine and compare the density value of pre- and post-1982 pennies and to explain the difference between their densities. First each group separated the pennies into two groups, one with pre-82 and one with post-82 pennies.

Then we measured out 50 mL of water into a graduated cylinder. This was going to be used to find the volume of the pennies, using displacement. Displacement is where you add the objects to the water and difference between the original volume and the new volume is the volume of the object. We massed the pennies in groups of 5 up to 30. After massing each group of 5 we then put them in the graduated cylinder to find the volume. We then divided the volume from the mass of each set to find the density.


Based on the data that we collected, the pre-82 pennies seem to have a higher density. The pre-82 pennies had a total density of 50.095 and the post-82 pennies had a density of 53.545.
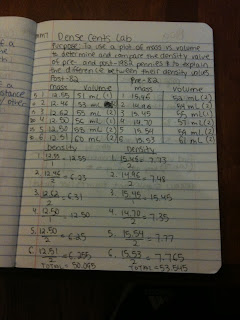
We concluded the class by returning to our seats. Mr. Henderson quickly read us a story about a man whose experience with nitric acid inspired him to keep experimenting with it. With little time left, we were instructed to turn to page 11 and complete the "Verbal Description of Change" and determine the Physical and Chemical properties. The answers are as followed: P, C, C, P, C, P, C, C, P.
Just as the bell was about to ring, Mr. H handed out the quizzes from last week and told us to check our answers and use it as a guide. He told us that tomorrow we could expect a lab about density and that our homework was finishing the 1.2 Webassign and our Delicious assignment.
 |
| Click to view video. |
 If you are trying to convert the numbers from scientific notation to the standard decimal notation, you have to move the decimal place and then fill in zeros. If there is a negative exponent, you will move the decimal point to the left ____ spaces. If there is a positive exponent, you will move the decimal point to the right ___ spaces. Like I said before, significant figures are still in effect for these problems.
If you are trying to convert the numbers from scientific notation to the standard decimal notation, you have to move the decimal place and then fill in zeros. If there is a negative exponent, you will move the decimal point to the left ____ spaces. If there is a positive exponent, you will move the decimal point to the right ___ spaces. Like I said before, significant figures are still in effect for these problems. The last section (3) on page 8 in the unit packet is practice using scientific notation on a calculator. When entering the operations on the calculater, you have to make sure to include all parentheses in order for the answer to come out correct. There is a special "EE" button on the CAS calculators especially for scientific notation. After getting an answer on the calculator, you were then to put those answers back into scientific notation, but more simplified than the original.
The last section (3) on page 8 in the unit packet is practice using scientific notation on a calculator. When entering the operations on the calculater, you have to make sure to include all parentheses in order for the answer to come out correct. There is a special "EE" button on the CAS calculators especially for scientific notation. After getting an answer on the calculator, you were then to put those answers back into scientific notation, but more simplified than the original.  The last thing we talked about in class was chemical and physical properties. A chemical property is a property of a substance which describes how the substance interacts (reacts) with another substance. A physical property is a property of a substance which describes the substance without describing its interaction with other substances. This was followed up by a demo lab where Mr. Henderson heated a colorless test tube over a methane burner. The test tube then turned a purplish color and took solid iodine and changed it to a gaseous iodine. This was used during the process of sublimation which includes freezing, melting, boiling, etc.
The last thing we talked about in class was chemical and physical properties. A chemical property is a property of a substance which describes how the substance interacts (reacts) with another substance. A physical property is a property of a substance which describes the substance without describing its interaction with other substances. This was followed up by a demo lab where Mr. Henderson heated a colorless test tube over a methane burner. The test tube then turned a purplish color and took solid iodine and changed it to a gaseous iodine. This was used during the process of sublimation which includes freezing, melting, boiling, etc.